Class 10 IT 402 Sample Pre-Board QP 2026 with Solution
Class 10 IT 402 Sample Pre-Board QP with Solution - SET 1
Class 12 IP 065 Sample Pre-Board QP 2026
The Class 12 Informatics Practices (IP) Sample Pre-Board Question Paper 2026 is designed to help…
Class 12 CS 083 Sample Pre-Board QP 2026 with Solution
Preparing for the Class 12 Computer Science (Code 083) board examination becomes much easier when…
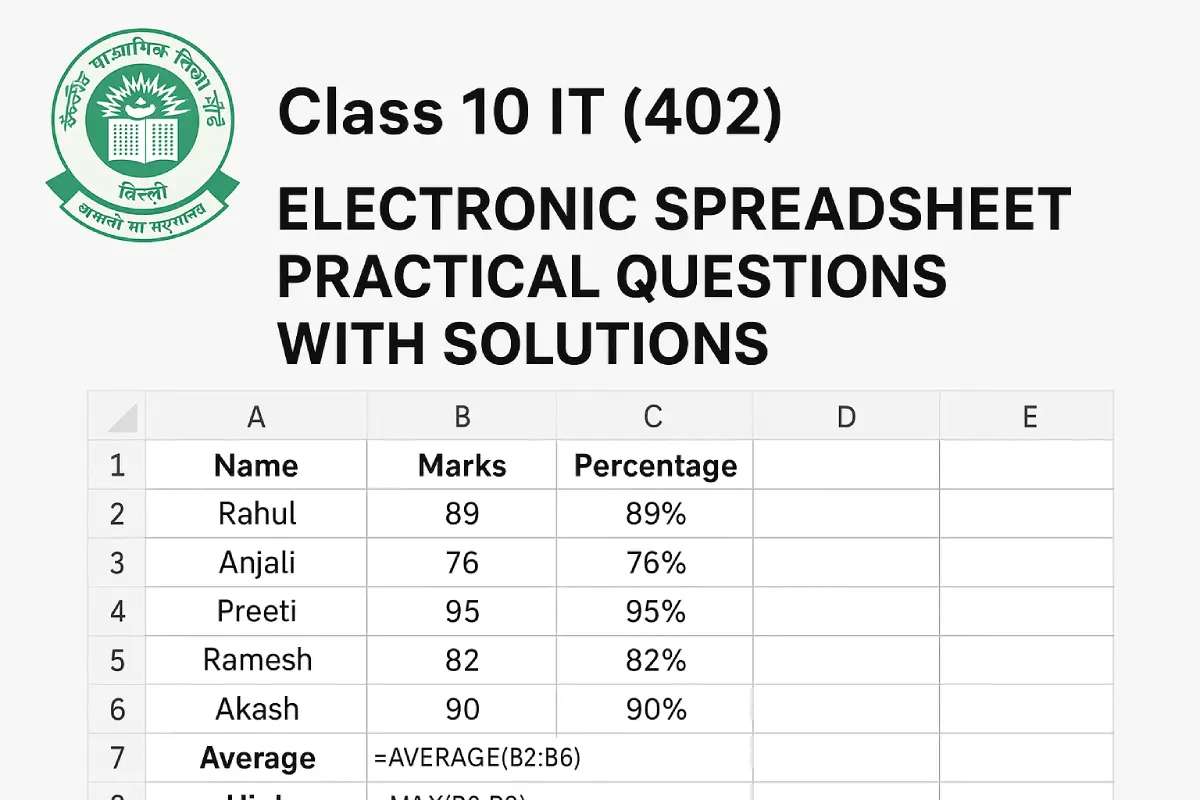
Class 10 IT (402) – Electronic Spreadsheet Practical Questions with Solution
1. Consolidate Data You have monthly sales data for January, February, and March in three…
Class 10 IT (402) – Digital Documentation Practical Questions with Solution
Digital Documentation Practical File Questions Practical Question 1: Using MS Word, create a well-formatted Resume…
Class 10 IT Code 402 Sample Question Paper 2026 with Solution
View CBSE Class 10 IT 402 Sample QP 2025-26 with Solution
Class 10 AI Code 417 Sample Question Paper 2026 with Solution
Class 10 AI Code 417 Sample Question Paper 2026 with Solution
Class 10 AI Code 417 Sample Question Paper 2024 with Solution
Class 10 AI Code 417 Sample Question Paper 2024 with Solution
RSMSSB Patwari 2025 Shift 2 Question Paper with Solution
The RSMSSB Patwari 2025 Shift 2 Question Paper with Answer Key is a valuable resource…
Class 9 AI Code 417 Sample Question Paper 1
Prepare effectively for your CBSE Class 9 Artificial Intelligence Board Exam 2026 with this official-style…